AI Bio 소식
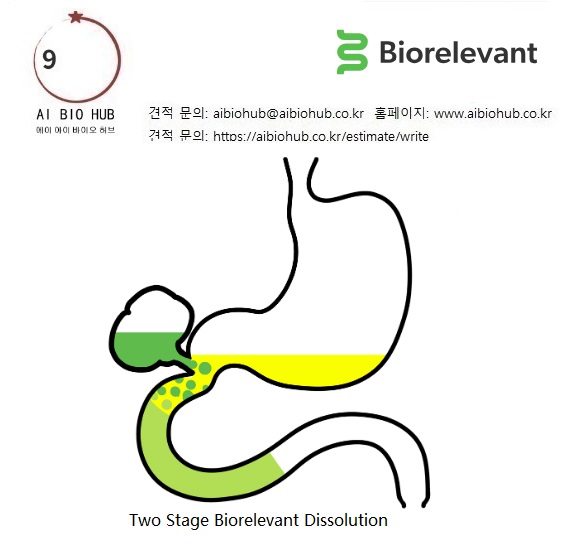
[Biorelevant 대리점 에이아이바이오허브]Two Stage Biorelevant Dissolution
- AI바이오허브 오래 전 2025.09.03 18:16 제품소개 인기
-
130
0
Biorelevant Experiment
Two Stage Biorelevant Dissolution
Simulate how a drug product behaves when it contacts the stomach fluid (FaSSGF) and then when it is converted into intestinal fluid (FaSSIF)
Particularly important for basic drugs with a higher solubility at acidic stomach pH compared to higher intestinal pH
Results will reveal the drug’s tendency to either supersaturate or precipitate
Results can help formulation development and optimisation
Two stage biorelevant dissolution is also referred to as a “biorelevant transfer test”. The method is designed to test how a drug product behaves when in contact with simulated gastric fluid (FaSSGF) which is subsequently converted into simulated intestinal fluid (FaSSIF). This in vitro experiment simulates the in vivo process when fasted stomach fluid containing a drug product is converted to fasted intestinal fluid. For immediate release formulations of basic drug, the two stage biorelevant dissolution test is conducted in USP 2 apparatus.
The test is mainly used during the development of drug products of water insoluble bases where solubility of the drug is higher in the gastric fluid than the intestinal fluid. The results reveal insights into precipitation or supersaturation as the pH is shifted from stomach to intestinal pH. It is a similar principle to the USP two-tier dissolution test described in USP Chapter <711> (Method A Procedure). However, the significant difference is that the acid and buffer stages used are biorelevant. Two media need to be prepared: FaSSGF and Two Stage FaSSIF (in effect 'concentrated' FaSSIF). Dissolution is first carried out in FaSSGF and continues as Two Stage FaSSIF is then added to make FaSSIF.
견적 문의: aibiohub@aibiohub.co.kr 홈페이지: www.aibiohub.co.kr
견적 문의: https://aibiohub.co.kr/estimate/write
- 이전글[Chemicea 대리점 에이아이바이오허브]6-Methylheptyl methacrylate(CP-A249001)2025.09.03
- 다음글[Biochemazone 대리점 에이아이바이오허브]Artificial Human Urine Based on Hydration Color Chart(BZ510)2025.09.03
댓글목록
등록된 댓글이 없습니다.