AI Bio 소식
[Medkoo 대리점 에이아이바이오허브]Deleobuvir(319692)
- AI바이오허브 오래 전 2025.08.27 23:11 제품소개 인기
-
123
0
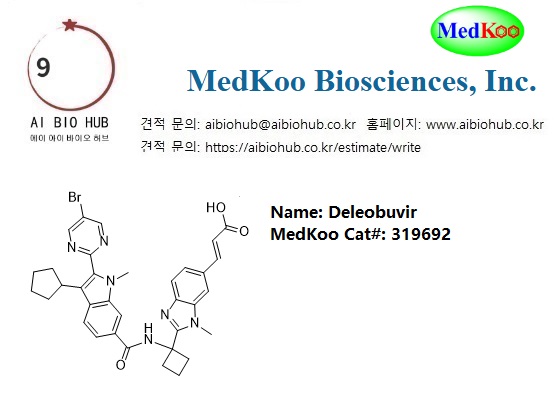
Deleobuvir
MedKoo Cat#: 319692
Deleobuvir, also known as BI207127, a non-nucleoside hepatitis C virus NS5B polymerase inhibitor for the treatment of hepatitis C. Deleobuvir was tested in combination regimens with pegylated interferon and ribavirin, and in interferon-free regimens with other direct-acting antiviral agents including faldaprevir. Deleobuvir showed that a triple combination of deleobuvir, faldaprevir, and ribavirin performed well in HCV genotype 1b patients. Efficacy fell below 50%, however, for dual regimens without ribavirin and for genotype 1a patients. In December 2013, deleobuvir was discontinued since recent findings from phase III trials did not suggest sufficient efficacy.
MedKoo Cat#: 319692
Name: Deleobuvir
CAS#: 863884-77-9 (free base)
Chemical Formula: C34H33BrN6O3
Exact Mass: 652.1798
Molecular Weight: 653.58
Elemental Analysis: C, 62.48; H, 5.09; Br, 12.23; N, 12.86; O, 7.34
Related CAS #:1370023-80-5 (sodium) 863884-77-9 (free base)
Synonym:BI207127; BI-207127; BI 207127; Deleobuvir; BI-207127NA; BI-207127Na
IUPAC/Chemical Name:(E)-3-(2-(1-(2-(5-bromopyrimidin-2-yl)-3-cyclopentyl-1-methyl-1H-indole-6-carboxamido)cyclobutyl)-1-methyl-1H-benzo[d]imidazol-6-yl)acrylic acid
InChi Key:BMAIGAHXAJEULY-UKTHLTGXSA-N
InChi Code:InChI=1S/C34H33BrN6O3/c1-40-26-17-22(10-11-24(26)29(21-6-3-4-7-21)30(40)31-36-18-23(35)19-37-31)32(44)39-34(14-5-15-34)33-38-25-12-8-20(9-13-28(42)43)16-27(25)41(33)2/h8-13,16-19,21H,3-7,14-15H2,1-2H3,(H,39,44)(H,42,43)/b13-9+
SMILES Code:O=C(O)/C=C/C1=CC=C2N=C(C3(NC(C4=CC5=C(C=C4)C(C6CCCC6)=C(C7=NC=C(Br)C=N7)N5C)=O)CCC3)N(C)C2=C1
Appearance:Solid powder
Purity:>98% (or refer to the Certificate of Analysis)
Shipping Condition:Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs.
Storage Condition:Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years).
Solubility:Soluble in DMSO, not in water
Shelf Life:>2 years if stored properly
Drug Formulation:This drug may be formulated in DMSO
Stock Solution Storage:0 - 4 C for short term (days to weeks), or -20 C for long term (months).
HS Tariff Code:2934.99.9001
Related CAS#
1370023-80-5 (Deleobuvir Sodium)
863884-77-9 (Deleobuvir)
Biological target:Deleobuvir (BI 207127) is a potent non-nucleoside hepatitis C virus (HCV) NS5B polymerase inhibitor.
In vitro activity:TBD
In vivo activity:TBD
REFERENCES:
1: Sane RS, Ramsden D, Sabo JP, Cooper C, Rowland L, Ting N, Whitcher-Johnstone A, Tweedie DJ. Contribution of major metabolites towards complex drug-drug interactions of deleobuvir: in vitro predictions and in vivo outcomes. Drug Metab Dispos. 2015 Dec 18. pii: dmd.115.066985. [Epub ahead of print] PubMed PMID: 26684498.
2: Asselah T, Zeuzem S, Soriano V, Bronowicki JP, Lohse AW, Müllhaupt B, Schuchmann M, Bourlière M, Buti M, Roberts SK, Gane EJ, Stern JO, Voss F, Baum P, Gallivan JP, Böcher WO, Mensa FJ. ITPA Genotypes Predict Anemia but Do Not Affect Virological Response with Interferon-Free Faldaprevir, Deleobuvir, and Ribavirin for HCV Infection. PLoS One. 2015 Dec 9;10(12):e0144004. doi: 10.1371/journal.pone.0144004. eCollection 2015. PubMed PMID: 26650626; PubMed Central PMCID: PMC4674133.
3: McCabe M, Sane RS, Keith-Luzzi M, Xu J, King I, Whitcher-Johnstone A, Johnstone N, Tweedie DJ, Li Y. Defining the Role of Gut Bacteria in the Metabolism of Deleobuvir: In Vitro and In Vivo Studies. Drug Metab Dispos. 2015 Oct;43(10):1612-8. doi: 10.1124/dmd.115.064477. Epub 2015 Jun 11. PubMed PMID: 26068924.
4: Yatsuhashi H, Kodani N, Ugai H, Omata M. Open-label phase 2 study of faldaprevir, deleobuvir and ribavirin in Japanese treatment-naive patients with chronic hepatitis C virus genotype 1 infection. Hepatol Res. 2015 May 20. doi: 10.1111/hepr.12535. [Epub ahead of print] PubMed PMID: 25991083.
5: Latli B, Hrapchak M, Chevliakov M, Li G, Campbell S, Busacca CA, Senanayake CH. Synthesis of deleobuvir, a potent hepatitis C virus polymerase inhibitor, and its major metabolites labeled with carbon-13 and carbon-14. J Labelled Comp Radiopharm. 2015 May 30;58(6):250-60. doi: 10.1002/jlcr.3294. Epub 2015 May 11. PubMed PMID: 25964148.
6: Kanda T, Yokosuka O, Omata M. Faldaprevir for the treatment of hepatitis C. Int J Mol Sci. 2015 Mar 4;16(3):4985-96. doi: 10.3390/ijms16034985. Review. PubMed PMID: 25749475; PubMed Central PMCID: PMC4394460.
7: Zeuzem S, Soriano V, Asselah T, Gane EJ, Bronowicki JP, Angus P, Lohse AW, Stickel F, Müllhaupt B, Roberts S, Schuchmann M, Manns M, Bourlière M, Buti M, Stern JO, Gallivan JP, Voss F, Sabo JP, Böcher W, Mensa FJ; SOUND-C2 Study Group. Efficacy and safety of faldaprevir, deleobuvir, and ribavirin in treatment-naive patients with chronic hepatitis C virus infection and advanced liver fibrosis or cirrhosis. Antimicrob Agents Chemother. 2015 Feb;59(2):1282-91. doi: 10.1128/AAC.04383-14. Epub 2014 Dec 15. PubMed PMID: 25512403; PubMed Central PMCID: PMC4335863.
8: Chen LZ, Sabo JP, Philip E, Rowland L, Mao Y, Latli B, Ramsden D, Mandarino DA, Sane RS. Mass balance, metabolite profile, and in vitro-in vivo comparison of clearance pathways of deleobuvir, a hepatitis C virus polymerase inhibitor. Antimicrob Agents Chemother. 2015 Jan;59(1):25-37. doi: 10.1128/AAC.03861-14. Epub 2014 Oct 13. PubMed PMID: 25313217; PubMed Central PMCID: PMC4291358.
9: Rossotti R, Travi G, Pazzi A, Baiguera C, Morra E, Puoti M. Rapid clearance of HCV-related splenic marginal zone lymphoma under an interferon-free, NS3/NS4A inhibitor-based treatment. A case report. J Hepatol. 2015 Jan;62(1):234-7. doi: 10.1016/j.jhep.2014.09.031. Epub 2014 Oct 5. PubMed PMID: 25285757.
10: Zeuzem S, Dufour JF, Buti M, Soriano V, Buynak RJ, Mantry P, Taunk J, Stern JO, Vinisko R, Gallivan JP, Böcher W, Mensa FJ; SOUND-C3 study group. Interferon-free treatment of chronic hepatitis C with faldaprevir, deleobuvir and ribavirin: SOUND-C3, a Phase 2b study. Liver Int. 2015 Feb;35(2):417-21. doi: 10.1111/liv.12693. Epub 2014 Oct 16. PubMed PMID: 25263751.
11: Zhang Y, Lu BZ, Li G, Rodriguez S, Tan J, Wei HX, Liu J, Roschangar F, Ding F, Zhao W, Qu B, Reeves D, Grinberg N, Lee H, Heckmann G, Niemeier O, Brenner M, Tsantrizos Y, Beaulieu PL, Hossain A, Yee N, Farina V, Senanayake CH. A highly concise and convergent synthesis of HCV polymerase inhibitor Deleobuvir (BI 207127): application of a one-pot borylation-Suzuki coupling reaction. Org Lett. 2014 Sep 5;16(17):4558-61. doi: 10.1021/ol5021114. Epub 2014 Aug 8. PubMed PMID: 25105303.
12: Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, Böcher WO, Thimme R. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol. 2014 Sep;61(3):538-43. doi: 10.1016/j.jhep.2014.05.043. Epub 2014 Jun 4. PubMed PMID: 24905492.
13: Cheng R, Tu T, Shackel N, McCaughan GW. Advances in and the future of treatments for hepatitis C. Expert Rev Gastroenterol Hepatol. 2014 Aug;8(6):633-47. doi: 10.1586/17474124.2014.909725. Epub 2014 May 20. Review. PubMed PMID: 24846594.
14: Uhl P, Fricker G, Haberkorn U, Mier W. Current status in the therapy of liver diseases. Int J Mol Sci. 2014 Apr 30;15(5):7500-12. doi: 10.3390/ijms15057500. Review. PubMed PMID: 24786290; PubMed Central PMCID: PMC4057686.
15: De Clercq E. Current race in the development of DAAs (direct-acting antivirals) against HCV. Biochem Pharmacol. 2014 Jun 15;89(4):441-52. doi: 10.1016/j.bcp.2014.04.005. Epub 2014 Apr 13. PubMed PMID: 24735613.
16: LaPlante SR, Bös M, Brochu C, Chabot C, Coulombe R, Gillard JR, Jakalian A, Poirier M, Rancourt J, Stammers T, Thavonekham B, Beaulieu PL, Kukolj G, Tsantrizos YS. Conformation-based restrictions and scaffold replacements in the design of hepatitis C virus polymerase inhibitors: discovery of deleobuvir (BI 207127). J Med Chem. 2014 Mar 13;57(5):1845-54. doi: 10.1021/jm4011862. Epub 2013 Nov 11. PubMed PMID: 24159919.
17: Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Müllhaupt B, Schuchmann M, Bourlière M, Buti M, Roberts SK, Gane EJ, Stern JO, Vinisko R, Kukolj G, Gallivan JP, Böcher WO, Mensa FJ. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013 Aug 15;369(7):630-9. doi: 10.1056/NEJMoa1213557. PubMed PMID: 23944300.
18: Larrey D, Lohse AW, Trepo C, Bronowicki JP, Arastéh K, Bourlière M, Calleja JL, Stern JO, Nehmiz G, Abdallah N, Berger KL, Marquis M, Steffgen J, Kukolj G; BI 207127 Study Group. Antiviral effect, safety, and pharmacokinetics of five-day oral administration of Deleobuvir (BI 207127), an investigational hepatitis C virus RNA polymerase inhibitor, in patients with chronic hepatitis C. Antimicrob Agents Chemother. 2013 Oct;57(10):4727-35. doi: 10.1128/AAC.00565-13. Epub 2013 Jul 15. PubMed PMID: 23856779; PubMed Central PMCID: PMC3811456.
19: Zeuzem S, Asselah T, Angus P, Zarski JP, Larrey D, Müllhaupt B, Gane E, Schuchmann M, Lohse AW, Pol S, Bronowicki JP, Roberts S, Arasteh K, Zoulim F, Heim M, Stern JO, Nehmiz G, Kukolj G, Böcher WO, Mensa FJ. Faldaprevir (BI 201335), deleobuvir (BI 207127) and ribavirin oral therapy for treatment-naive HCV genotype 1: SOUND-C1 final results. Antivir Ther. 2013;18(8):1015-9. doi: 10.3851/IMP2567. Epub 2013 Apr 4. PubMed PMID: 23558093.
20: Zeuzem S, Asselah T, Angus P, Zarski JP, Larrey D, Müllhaupt B, Gane E, Schuchmann M, Lohse A, Pol S, Bronowicki JP, Roberts S, Arasteh K, Zoulim F, Heim M, Stern JO, Kukolj G, Nehmiz G, Haefner C, Boecher WO. Efficacy of the protease inhibitor BI 201335, polymerase inhibitor BI 207127, and ribavirin in patients with chronic HCV infection. Gastroenterology. 2011 Dec;141(6):2047-55; quiz e14. doi: 10.1053/j.gastro.2011.08.051. Epub 2011 Sep 16. PubMed PMID: 21925126.
견적 문의: aibiohub@aibiohub.co.kr 홈페이지: www.aibiohub.co.kr
견적 문의: https://aibiohub.co.kr/estimate/write
- 이전글[Chemicea 대리점 에이아이바이오허브]3-Methyl Adenine(CP-A352001)2025.08.28
- 다음글[Chemicea 대리점 에이아이바이오허브]Acebutolol EP Impurity C(CP-A94003)2025.08.27
댓글목록
등록된 댓글이 없습니다.