AI Bio 소식
[BOC Science 대리점 에이아이바이오허브]Doxofylline Impurity 1(B2694-470470 )
- AI바이오허브 오래 전 2025.10.11 00:12 제품소개
-
22
0
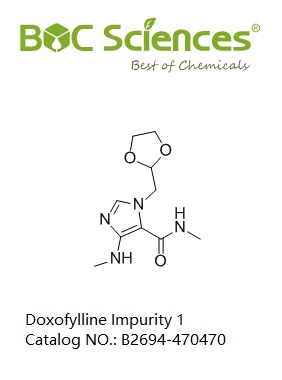
Doxofylline Impurity 1
Catalog NO.: B2694-470470
CAS NO.: 1429636-74-7
Brand: BOC Sciences
Category:Impurities
Tag:Doxofylline and Impurities
Molecular Formula:C10H16N4O3
Molecular Weight:240.26
Product Description:Doxofylline Impurity 1 is a Doxofylline impurity. Doxofylline is a phosphodiesterase inhibitor and a xanthine derivative drug for asthma.
Purity:> 95%
Synonyms:1-(1,3-Dioxolan-2-ylmethyl)-N-methyl-4-(methylamino)-1H-imidazole-5-carboxamide
IUPAC Name:3-(1,3-dioxolan-2-ylmethyl)-N-methyl-5-(methylamino)imidazole-4-carboxamide
PROPERTIES
Quantity:Milligrams-Grams
InChI Key:NZNGFFZEKHUMKT-UHFFFAOYSA-N
InChI:InChI=1S/C10H16N4O3/c1-11-9-8(10(15)12-2)14(6-13-9)5-7-16-3-4-17-7/h6-7,11H,3-5H2,1-2H3,(H,12,15)
SMILES:CNC1=C(N(C=N1)CC2OCCO2)C(=O)NC
Scheme 1-HPLC-MS analysis of Doxofylline (DFP) impurity 1
Doxofylline (DFP) and its process impurities were dissolved in methanol in specified proportions to create solutions (1000 μg/mL). In order to investigate linearity, precision, accuracy, quantification limits, and limits of detection, the solutions were suitably diluted with the mobile phase. The DFL specified concentration was determined to be 500 μg/mL.
Using a mobile phase made up of a combination of 10 mM ammonium acetate and acetonitrile in gradient mode of elution, chromatographic separation was accomplished on C18. After the mobile phase was newly made, it was sonicated for 15 minutes and filtered using a Millipore filter with a pore size of 0.45 μm. The separation process was ran for 30 minutes at 30 °C with a flow rate of 1 mL/min. The detection wavelength was set at 274 nm, and the injection volume was 20 μL.
The examination of impurities was conducted using the MS instrument, separation module pumps, auto sampler device, and a photodiode array detector. Positive way of detection with electrospraying ionization was employed. The curtain gas and nebulizer were nitrogen. Helium served as the collision gas and caused collision-induced dissociation. The following were the settings for the ion source: 325 °C for the dry temperature; 60 psi for the nebulizer gas; 5.0 L/min for the dry gas; 113.5 V for the capillary exit; 81.787 nA for the capillary current; 4000 nA for the corona current; 2100 V for the electro multiplier voltage; 400 °C for the vaporizer temperature; and 200 ms for the dwell duration.
HPLC chromatograms of Doxofyllineits impurities.HPLC chromatograms of Doxofylline (DFP) its impurities. (Rao R N., et al., 2013)
Reference
Rao R N., et al., Development and validation of a stability indicating assay of doxofylline by RP-HPLC: ESI-MS/MS, 1H and 13C NMR spectroscopic characterization of degradation products and process related impurities, Journal of pharmaceutical and biomedical analysis, 2013, 78: 92-99.
APPLICATION:
Doxofylline Impurity 1 is a particular impurity that might be found in doxofylline, a xanthine derivative used as a bronchodilator. Impurities in pharmaceuticals, such as doxofylline, are undesirable compounds that can form during the production or storage process.
Develop analytical methods: Doxofylline Impurity 1 is used for calibrating analytical instruments including high-performance liquid chromatography (HPLC) and gas chromatography (GC). Create calibration curves to aid in the quantification of contaminants in pharmaceutical goods by producing standard solutions of the impurity. Doxofylline Impurity 1 can be used as an internal standard to accurately quantify impurities in complicated mixtures. It aids in adjusting for changes in sample preparation and instrumental circumstances.
For stability studies: Doxofylline Impurity 1 can be used in forced degradation experiments to better understand the breakdown mechanisms of doxofylline. By subjecting doxofylline to various stress conditions (e.g., heat, light, pH extremes), the impurity might be identified and quantified, providing information on the drug's stability. When developing stability-indicating techniques, Doxofylline Impurity 1 is used to ensure that the analytical methods can reliably identify and quantify the impurity in the presence of the parent drug and other degradation products.
For chemical synthesis: Doxofylline Impurity 1's functional groups allow it to be used as an intermediary in the synthesis of more complicated compounds. The dioxolane ring can undergo ring-opening reactions, whereas the carboxamide can engage in a variety of condensation processes, making it an adaptable building block. The dioxolane moiety and imidazole ring may interact with polymers, affecting their physical characteristics. This chemical might be used as an addition to improve thermal stability, mechanical strength, and other properties of polymeric materials.
For impurity profiling: Establishing impurity libraries using Doxofylline Impurity 1 can help identify and quantify impurities in various batches of doxofylline. This enables consistent and high-quality pharmaceutical manufacture.
견적 문의: aibiohub@aibiohub.co.kr
홈페이지: www.aibiohub.co.kr
- 이전글[Angene Chemical 대리점 에이아이바이오허브]1H-Pyrrolo[3,4-b]pyridine, octahydro-1-methyl-(AG000YDD)2025.10.13
- 다음글[Biochemazone 대리점 에이아이바이오허브]Artificial Eccrine Perspiration – Stabilized 1000 ml (BZ113)2025.10.10
댓글목록
등록된 댓글이 없습니다.